We like to share product recommendations with you and hope you like them! Just to make you aware Water Filter Data may collect a small share of sales or other compensation from the links on this page.
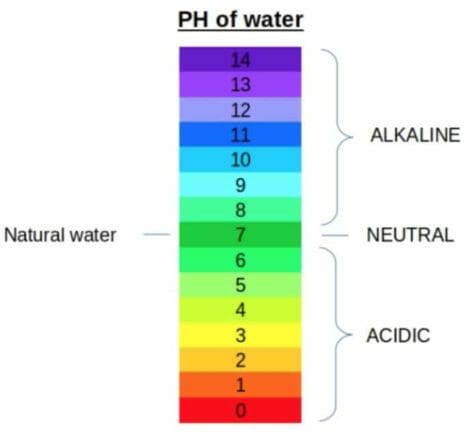
PH represents the water sample’s acidity. It is a measure of the potential activity of hydrogen ions (H+) within that sample. pH measurements can be on a scale from 0 to 14, with 7.0 being neutral, or unpolluted water. Anything with a pH below 7.0 is considered acid, while a pH above 7.0 a base (or alkaline). All organisms are subject to the amount of acidity of stream water and function best within a certain range.

How Does Water Become Acidic or Alkaline?
When it comes to water, it’s natural to have an acidic or alkaline condition. It’s essential to maintain a healthy pH balance in every component, including your mouth. For instance, if the acidic level goes below 5.5, it can lead to the growth of harmful bacteria in your mouth, leading to tooth decay.
This applies to your entire body, too. No wonder you need to ensure the right pH balance in the water you consume.
When water has a neutral Ph level, it tastes better. For maintaining a healthy body, you need to consume water with a pH level of around 7. At times, the pH level in water gets affected by pesticide contamination, acid rain, and the nature of minerals present in the rocks in your area. Even metal leaching from pipes may lead to changes in the pH level in the water.
Alkaline water, on the other hand, tastes bitter. The intensity depends largely on the level of alkalis present in it. This type of water can cause the corrosion of water pipes.
If the pH level crosses 9, it might corrode metals like brass, iron, zinc, copper, and aluminum. If your demographic zone has hard alkaline water, you might find it difficult to lather soap. Besides, the deposits might accumulate on your dishes. Acidic water tastes sour or metallic.
If the pH level of the water you’re using is less than 6.5, the toxic levels would contain metals like iron and manganese. Acidic water is harmful to your hardware, as it can break down the pipes and leach metals. In case you come across bluish stains on the pipes or your sinks, it indicates high acidic levels.
Possible Side Effects of Consuming Acidic Water
Healthcare professionals recommend not to consume acidic water. It contains high proportions of cadmium, chromium, lead, arsenic, zinc, copper, and nickel.
The heavy metal concentration can lead to various health issues. Heavy metal poisoning is common, leading to complications like organ damage, diarrhea, abdominal pain, nausea, and vomiting.
Unfiltered water containing high proportions of acid can also lead to weakness, chills, immune system suppression, and shortness of breath. However, the intensity of these conditions depends on different aspects, such as individual susceptibility, age, gender, and frequency of exposure to such water.
The water which you consume or use at home may become acidic naturally. Therefore, it’s imperative to use a good quality water filter and reduce the level of acidity.
They can effectively minimize the concentration of heavy metals, thereby providing pure drinking water. No wonder why most households use advanced water filters to get rid of the acidic or alkaline content.
Want To Test Your Water’s pH Without A pH Test Kit. What To Do?
Any water with low pH could dissolve poisonous chemicals. Such water is far from being safe for drinking. If you believe you have water with similar indications, it is advisable to know some simple tests you can do and get fast results valuable for your household.
Read what the US EPA (United Nations Environmental Protection Agency) says about testing your water.
Although water pH test kits usually have indicator drops, pH test strips, and a pH test meter, you can do a pH water test without such a testing kit. Please be aware that the upcoming tests are not intended for commercial testing of products that should enter sales cycles.
Test Your Water Using The Red Cabbage Test
In moments when you don’t have a testing kit, buy a red cabbage and a bottle of distilled water. These two items are enough for you to create a pH indicator. A water-soluble pigment flavin (an anthocyanin) the cabbage has is responsible for the red color you will get. Only two items are sufficient to get quick and effective results when testing your water.
Cut your red cabbage and put it in a glass bowl. Boil two cups of distilled water. Next, pour the boiled water in the bowl with the cabbage, fully covering it. Stir occasionally and leave the cabbage in the distilled boiled water for around 30 minutes. The chemical reaction in the cabbage will cause the pigment molecules to alter the distilled water’ color. Straining off the liquid with a purplish red color will work as your pH indicator solution. Pour the resulting liquid in a different plastic or container. Now, put a few pH indicator solution drops in that container.
The watercolor will change and will turn into either a red/pink (acidic), purple (neutral) or bluish-green, blue, or greenish-yellow color (alkaline). Matching your color against a color chart will give you a numerical value on the pH scale, telling you exactly what water you have in your place.
Make Your Own pH Paper

The process of making pH paper is quite simple. Get dry litmus powder either from a chemical supply store or online. Dissolve the litmus in water, while making sure you mix them well. Use heat in case you see the powder is not dissolving well. The solution should have a violet-blue color.
Place the white acid-free paper in the litmus solution. Don’t leave it to soak in the water – keep it enough, so you are sure it is thoroughly coated. Afterward, allow it to dry in the open air, but make sure not to expose it to basic or acidic vapors. Storing them in a dry, dark place will help you in preventing contamination or bleaching.
Once dry, use the litmus paper to test for acidity. Blue litmus papers will turn red when there is acid presence. No change symbolizes a neutral or basic solution, but not acidic.
Best Way To Exame pH Level
A digital pH meter is the most accurate way to test pH presence in your water. Testing with a meter will reduce the chance for error to a minimum, as well as any other type of human error. As people see colors differently, not everyone will choose the correct color, therefore reaching a different conclusion. The pH meter also removes the likelihood of contamination by mishandling test strips, using outdated strips, or even storage strips issues that might influence the overall reading accuracy.
A pH meter doesn’t depend on color differences as it presents the numerical pH value. It measures the total amount of hydrogen-ion in water by setting a glass electrode in the water. To be sure of the reading, verify the meter accuracy with a pH substance before the test takes place. After the meter verification is done and before testing the water, clean the probe tip with double deionized water, and dry it with a clean cloth or tissue.
Pour your test water in a clean container, and wait a few minutes for it to stabilize the temperature. As the temperature affects the pH, you should determine the water temperature with a thermometer. It should match the meter’s default temperature, so enter the water temperature into the meter. Then, drop the probe in the water sample, completely covering the probe tip. Wait for the meter display to stabilize and see the outcome.
For a visual representation of the overall process, feel free to check out the following guide.

How To Neutralize The pH In Your Water?
- Acidic substances are applied when neutralizing base alkaline water, while base alkaline substances during neutralization of acidic water. Some of the most common ways to do this in your home include:
- Adding a filter at the water’s entry point. The calcium carbonate, a carbonic salt of calcium, is the substance used for the neutralization of alkaline water. On the other side of the spectrum, it is a synthetic magnesium oxide substance that will neutralize acidic water.
- Installing an injection system at the water entrance. Soda ash, which is acetic acid similar to the one found in vinegar, citric acid, or alum, is applied to neutralize high alkaline levels of water. However, the injection systems require more maintenance compared to a filtering system.
- Setting up a water distillation system that can neutralize the acidic pH of drinking water. The distiller will heat and eliminate any acidic particles, creating acid-free steam.
- Implementation of electrolysis within a water ionizer system that will separate the alkaline and the acidic water parts. In here, the alkaline water is used for drinking, while the acidic water for washing and cleaning. Ionizers are more expensive than neutralizing filters and neutralizing solutions.
Conclusion
Every liquid substance, no matter if it is just water, just, or even our saliva, has a pH level. Many times, testing the ph level, especially the water pH is important for various reasons. Luckily, this can be tested in many different ways. The options shared in this guide are easy, cheap, and quite suitable for home testing.
Want to learn more about water filters? Check out our full list of filter types in our guide .

Austin is the lead water consultant and blogger for Water Filter Data. With 10 years of experience in the water quality industry, Austin can instantly pinpoint the cure and the cause for the water pollutant.



